Document Type : Original Research Article
Author
Department of Chemistry, Benue State University, Makurdi, Nigeria
Abstract
Binary complexes of Cu(II) and Mn(II) with cysteine and cephalexin have been studied potentiometrically at about 27 in aqueous medium using Irving-Rossotti titration technique for proton-ligand (P-L) and metal-ligand (M-L) stability constants evaluation. The proton-ligand stability constants for cysteine and cephalexin were: log K1H 8.4 (pKa for thiol group), log K2H 10.7 (pKa for NH2-group), log K1H 5.2 (pKa for carboxyl group), and log K2H 6.82 (pKa for amine group), respectively. The binary metal-ligand stability constant (logK) values for 1:1 were found in the order of Cu(II) > Mn(II) for both ligands in conformity to the Irving–Williams order of the divalent transition metals of the period 4. Though the redox-active nature of Cu(II)-Cysteine atmosphere likely compromised the Cu(II)-Cysteine stability constant. By and large, M-L stability constants of Mn and Cu with cysteine and cephalexin have been re-established. This finding agreed with previous studies claimed that cysteine is fit for use in transporting target metals. In general, the studies have strengthened the idea that drugs and amino acids are able to interact with metals in our body system to a specific extent in terms binding constant.
Graphical Abstract
Keywords
Main Subjects
Introduction
Amino acids are essential integral part of foundation of living organisms. Amino acids unite to form proteins which are abundant in our body system. The proteins interaction with metals present in our bodies is then inevitable. Thus, the study metal-ligand constants of amino acids and drugs are essential for their potential applications [1] and gaining insight into the metal-protein and metal-drug activities in the body. Amino acids bind to metals ions via their amino (NH2), carboxylic (COO-) groups, etc. [2,3]. Thus, they are used as antidote to metal poisoning [1] and as chelants in general [4]. Many chelating agents have been used to move metals to or away from target sites because of their ability to form strong bonds with different metal ions. However, good number of these traditional chelating agents have been reported to be toxic, non-biodegradable, and rigid towards the recovery of ligated metal ions [5]. The inherent drawbacks with these chelators necessitate a search for their alternatives [6] which are nontoxic and flexible for recovering bound metal ions. The therapeutics we used are influenced by the presence of metals in our biological fluids [7]. Thus, the study of metal complexes of biologically active ligands can define the degree of the formation constant between the metal and the amino acids, or the drug. In addition, these studies can identify the specific atoms or groups that are responsible for binding to metal ions, or are used in removal of toxic heavy metal ions [1]. In addition, the extend of metal-ligand complex formation is therapeutically useful because the function of a drug is related to its mode- i.e. either in free or complexed form [8]. Furthermore, important physiological reactions are facilitated by Cu-containing enzymes. In general, Cu(II) is known to form complexes with proteins, peptides, and enzymes in the living organisms [1]. Manganese (Mn) facilitates the formation of connective tissue, bones, blood clotting factors, and sex hormones. Mn again helps in fat and carbohydrate metabolism, calcium absorption, blood sugar regulation, and for normal brain and nerve function. Many methods exist for the study of proton-ligand and metal-ligand stability constants. However, as a result of accuracy and reliability, pH-metry technique is frequently used [7,9].
Some of the following precautions are taken during the determination of M-L formation constants: The metal concentration should be lesser than that of ligand so as to prevent hydrolysis of metal ions; secondly, the ionic strength should be observed at 0.2 M to prevent the tendency of anionic species and the cationic species or strong electrolytes ion pair formation [8]. Furthermore, it has been revealed that the reaction of metal ion with high covalent index with ligand of high polarizability increases the M-L stability constants [6]. This study reports binary stability constants studies of Cu- and Mn - complexes with cysteine and cephalexin using Calvin-Bjerrum titration (10) technique as applied by Irving & Rossotti (10), to enable us validate previous claim about the metal-cysteine complexes of these metal ions. Of course, the work will strengthen the idea that drugs and amino acids/ proteins interact with metals in our body system to a specific extent in terms of binding constant. Cysteine and cephalexin chosen here have similar functional groups (thiol, C=O, COOH, and amine).
Experimental
Materials/ apparatus/ equipment
Cephalexin and cysteine were obtained without further purification. A fresh sample was taken and a solution was prepared for each titration to overcome hydrolysis. The metal salts and other reagents were all of analytical grade. Stock solutions of the metal salts, HCl, and NaOH were prepared in double-distilled water. The potentiometric measurements were carried out by pH-meter. The meter was calibrated using standard buffers of pH 4.00 and pH 9.00.
Potentiometric studies procedure
The potentiometric studies were carried by using Irving and Rossoti titration technique [7-8,10]. These solutions were prepared and titrated against 0.04 M NaOH free of CO2 at 27 . (a) 3 mL 0.04 M HCl (b) Solution (a) + 4 mL 0.03 M cysteine/ cephalexin (c) Solution (b) + 2 mL 0.04 M metal (Cu(II) and Mn (II)) chloride solutions, respectively. The total volume in each run of the above titration was kept at 50 mL. Thus, log K values were subsequently determined using the Calvin-Bjerrum method as adopted by Irving and Rossotti [1,7].
Results and Discussion
The irving-rossotti potentiometric titration
The proton–ligand formation constants of the cephalexin and cysteine and the binding constants of their complexes with Cu(II) and Mn(II) have been determined in aqueous medium at 27 . Therefore, the potentiometric titration curves of cysteine and cephalexin and their Cu(II) and Mn(II) complexes are presented in Fig. 1 and 3, respectively. In addition, no hydroxo complexes were found since there was no precipitate found during the titration [6].
Proton-ligand stability constant
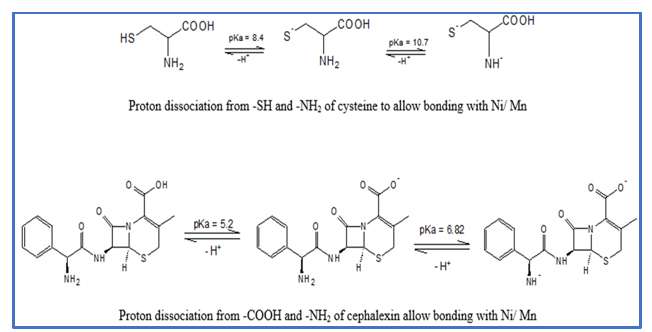
From Figures 1 and 3 (at least at pH above 7 in the case of cephalexin), the titration curves indicate that the ligand and metal curves are below the acid titration curve in each case. This change in the curve trend is due to the release of proton from the ligands (cysteine and cephalexin) [1,6-8]. Hence, the cysteine and cephalexin bonded to the metals via displacement of proton(s) [6,10]. In addition, in Figure 1, the cysteine released protons right from the pH of 2.8 and above. Whereas, for cephalexin (whose pKa are 5.2 and 6.82) released proton at pH of about 7 and above. General understanding is that a molecule is often protonated at pH less than its pKa value. The degree of formation of the proton complex (nA) was evaluated according to Equation 1 [11]. Y = number of replaceable hydrogen ion; V° = total volume 50 mL; V1 = volume of alkali required by the acid; V2 = volume of alkali used by acid and ligand; No = concentration of alkali; E° = total strength of acid; and TcL° = total concentration of ligand. Therefore, to find the proton-ligand stability constant (pKa), integral method was applied on the Equations 2 and 3 [12]. From this approach if we put the value of nA = 0.5 in Equation 2, then log K2H = pH. Also, when 1.5 is taken for nA in the Equation 3, we obtain log K1H = pH. Consequently, if a plot is made for nA versus pH, the resulting values of pH for nA = 0.5 and 1.5 becomes log K2H and log K1H, respectively [1,7]. Therefore, from the plots of nA versus pH (see Figure 2 for cysteine and Figure 4 for cephalexin), the values of log K1H (first proton-ligand formation constant) and log K2H (the second proton-ligand formation constant) were evaluated accordingly. It was observed for the cysteine - see Scheme 1 - that the log K1H 8.4 (lower pKa value) corresponds to the proton from thiol group and log K2H 10.7 (higher pKa value) is associated to the proton of the NH2-group. On the otherhand, log K1H 5.2 (lower pKa value) corresponds to the proton from -COOH group and log K2H 6.82 (higher pKa value) is associated to the proton of the NH2-group in the cephalexin as described in Scheme 2. The pKa value for the COOH group in the cysteine was not observed due to the condition of the experiment. Thus, the thiol group got (of the cysteine) involved in the complex formation [7,13]; in addition to the COOH and the amine groups (in the cysteine and the cephalexin). According to Al-Mohaimeed and Alothman, S-methylcysteine exhibits pKa value of 8.65. They were of the opinion that the thiol group contributes in the complex formation process [14].
Metal-ligand stability constant
The Irving and Rossotti Potentiometric technique was used for the determination of the binary M-L stability constant [8]. As earlier observed that there was drop in pH of the titration curve of the free ligand to that of the ligand + M indicating the complex formation. Hence, the average number of ligands attached per complex ion ( ) were evaluated by Equation 4 [8], where Vn = volume of alkali used for acid + ligand + metal ion titration; TcMo = total concentration of the metal ion, the rest of the terms are familiar from Equation 1. The free ligand exponent, pL were then obtained by applying the Equations 5 [1,7-8]. The log K1 and log K2 can be expressed as given in Equations 6 and 7, respectively; from the point wise method for binary M-L stability constant determination [12]. By the consideration of the integral method and taking = 0.5 in Equation 6; logK1 = pL. In a similar way, logK2 = pL (using the Equation 7) when = 1.5. In a nutshell, in the plot of vs pL, the corresponding values of pL for = 0.5, or 1.5 gives log K1 and log K2, respectively [1,7-8]. Subsequently, in this work, plots of pL vs were made and the binary M-L stability constants were deduced, as presented in Table 1. However, the stability constants for = 1.5 (M: L; 1: 2) were not found at the conditions of these experiments. The 1:2; M: L complex are often less stable in relation to 1: 1 counterpart [8]. The values of the stability constants, (1: 1, M: L) were 7.50 and 33.2 for Mn(II) and Cu(II), respectively for cysteine. In the case of cephalexin, the M: L stability constants were 3.2 and 3.4 for Mn(II) and Cu(II), respectively. The order of the stability constants is: Cu(II) > Mn(II) in both cysteine and cephalexin which conforms to the Irving–Williams order of the M2+ metal ions of 3d series [7][15]. In addition, the absence of 1: 2 (M: L) complexes in this work may be due to the experimental conditions such as the concentration of ligand, and ionic strength, etc. [7]. In general, Cu(II) -Cys complexes are found to be very unstable [16] because Cu(II) has high tendency to oxidize cysteine [16]. Therefore, the redox-active phenomenon of Cu-Cys environ can yield range of unusual coordination species [17]. In essence, the binary M-L stability constant of the Cu(II) – Cys as seen in this work is likely compromised.
Moreover, Adam et al. [10] reported the binary stability constants of Cu(II)-leucine and Ni(II)-leucine as 8.15 and 5.87, respectively, and 8.12 and 5.78 for Cu(II)- isoleucine and Ni(II)-isoleucine, respectively [10]. On the otherhand, Ishola et al. [6] found that the binary formation constants of Cu(II) - L-tyrosine, Co(II) - L-tyrosine, and Pb(II) - L-tyrosine were 6.40, 4.20, and 6.98, respectively [6]. Similarly, M-L stability constants evaluation of Cu(II) and Mn(II) complexes of chlorosubstituted pyrazoles and isoxazoles by Calvin Bjerrum titration as applied by Irving-Rossotti were gotten within the range of 5.343-3.644 [9]. These values are comparable to the ones we found here. Again, the potentiometric determination of stability constant of Fe(III) - pyrazinamide was carried out by Kosasy et al. [8]. They observed M-L stability constants of 2.75 and 1.6 for 1: 1 and 1:2 complexes, respectively. Binary and ternary complexes of Fe(III), Pb(II), Co(II), Al(III), La(III), Sr(II), Cr(III), Ti(II), and Zr(II)) with sulphathiazole and glycine were potentiometrically studied [7]. While Al(III) and Zr(IV) ions formed M: L complexes of 1:1, 1:2, and 1:3 (7); Zr(IV), Sr(II), Al(III), Fe(III), Th(IV), and Pb(II) produced 1:1 and 1:2, M: L complexes. On the contrary, the report of Esmaielzadeh and Mashhadiagha has it that Co(II), Cr(III), Ti(II) and La(III) only formed 1:1, M: L complexes with Schiff base ligand [18]; as similarly seen in this current report.
The stability constants in general should be inversely proportional to metal ion radius (RI) for M-L complexes of same metal ion charge with similar electronic configuration [15]. However, for metal ions of different groups this is untrue. Thus, the Cu(II) complexes have higher stability constants because Cu(II) has smaller ionic radius than Mn(II). Furthermore, M-L stability constants are noted to be directly proportional to the EN, Z, and IP of the metal, as described in Table 1. Moreover, increasing the EN of the metal ions will decrease the EN difference between the metal atom and the donor atom of the ligand. Thus, the M-L bond would have more covalent character, resulting into greater stability of the metal complex [15].
Conclusion
Therefore, the binary complexes of Cu(II) and Mn(II) with cysteine and cephalexin have been studied potentiometrically at about 27 in aqueous medium using Irving-Rossotti titration technique for P-L and M-L stability constants evaluation. The P-L stability constants for cysteine and cephalexin were: log K1H 8.4, log K2H 10.7; and log K1H 5.2, log K2H 6.82, respectively. The binary M-L stability constant (logK) values for 1:1 were found in the order of Cu(II) > Mn(II) for both ligands in conformity to the Irving-Williams order of the M2+ transition metals of the period 4. Though the redox-active nature of Cu(II)-Cysteine atmosphere likely compromised the Cu(II)-Cysteine stability constant. By and large, M-L stability constants of Mn and Cu with cysteine and cephalexin have been re-established. This finding agreed with previous claimed that cysteine is fit for use in transporting target metals. In general, the studies have strengthened the idea that drugs and amino acids are able to interact with metals in our body system to a specific extent in terms binding constant.
Acknowledgements
The authors are thankful to the Department of Chemistry, Benue State University, Makurdi for making facilities available for use for this work.
ORCID
Kaana Asemave
https://orcid.org/0000-0002-2870-3547
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Declarations
Conflict of interest: The authors have no relevant financial or non-financial interests to disclose.
Ethical approval: Not applicable.
Consent to participate: Not applicable.
Consent for publication: Not applicable
--------------------------------------------------------------------------------------------------------------------------------------------------
OPEN ACCESS
©2024 The author(s). This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit: http://creativecommons.org/licenses/by/4.0/
PUBLISHER NOTE
Sami Publishing Company remains neutral concerning jurisdictional claims in published maps and institutional affiliations.
CURRENT PUBLISHER
Sami Publishing Company
- Belkher NA, Al-abbas AA, Zidan M. Potentiometric Studies on Stability Constant of the Complexes of Some Essential Transition Metal Ions with L-Valine. Journal of Pure & Applied Sciences. 2019;18(3):59–63. [Google Scholar]
- Asemave K, Anhwange BA, Anom TJ. Antibacterial studies of leucine complexes of Fe (III) and Cu (II). International Journal of Science and Research (IJSR), 2015;4(1):1527–9. [Google Scholar], [Publisher]
- Asemave K, Yiase SG, Adejo SO. Kinetics and Mechanism of Substitution Reaction of trans-Dichlorobis (ethylenediammine) cobalt (III) chloride with Cysteine. International Journal of Modern Organic Chemistry. 2012;1(1):1–9. [Google Scholar], [Publisher]
- Asemave K. Greener Chelators for Recovery of Metals and Other Applications. Org Med Chem Int J. 2018;6(4):001–11. [Crossref], [Google Scholar], [Publisher]
- Asemave K. Biobased Lipophilic Chelating Agents and their Applications in Metals Recovery. University of York, UK; 2016. [Crossref], [Google Scholar], [Publisher]
- Ishola KT, Oladipo MA, Odedokun OA, Olanipekun OT. Potentiometric Studies of Stability Constants and Speciation of Binary and Ternary Complexes of Metal (II) Ions with Amino Acids and Thiobarbituric Acid. American Journal of Applied Chemistry. 2020;8(1):23–30. [Crossref], [Google Scholar], [Publisher]
- Al-Rashdi AA, Naggar AH, Farghaly OA, Mauof HA, Ekshiba AA. Potentiometric Determination of Stability Constants of Sulphathiazole and Glycine-Metal Complexes. American journal of analytical chemistry. 2018;9:99–112. [Crossref], [Google Scholar], [Publisher]
- Kosasy AME, Ghonim OAA, Ayada MF, Abdel-Fattah LE. Spectrophotometric and potentiometric determination of the stability constant of pyrazinamide-Fe(III) binary complex. Anal Chem (An Indian Journal). 2011;10(3):165–169. [Crossref], [Google Scholar].
- Nandurkar PS, Rathore MM. Study of proton-ligand and metal-ligand stability constants of Cu (II) and Mn (II) complexes with chlorosubstituted pyrazoles and isozoles in 80% DMF-water solvent using pHmeter. Int J ChemTech Res. 2017;10(15):204–12. [Crossref], [Google Scholar].
- Adam A, Verma S, Seth G. Stability Constants of Mixed Ligand Complexes of Cu(II) and Ni(II) with Some Amino Acids and Phosphates. Journal of Chemistry. 2011;8(S1):S404–8. [Crossref], [Google Scholar], [Publisher]
- Gayakwad SV., Maulage SB, Wankhede DS. PH- Metric Study of Mixed Ligand Complexes of Vanadium with Catechol as Primary and Amino Acids as Secondary Ligands. Res J Pharm Biol Chem Sci. 2017;8(3):1647–52. [Crossref], [Google Scholar], [Publisher]
- Janrao DM, Pathan J, Kayande DD, Mulla JJ. An over view of potentiometric determination of stability constants of metal complexes. Sci Revs Chem Commun. 2014;4(1):11–24. [Google Scholar], [Publisher]
- Alturigi AS, Anazy MM, AlFarraj ES, Ammar RA. Stability Constants of Mixed Ligand complexes of Cu(II) and Atenolol with L-methionine/ L-Cysteine/ L-penicillamine and S-methyl-L-cysteine. Int Jo Electrochem Sci. 2020;15:11275–82. [Crossref], [Google Scholar], [Publisher]
- Al-Mohaimeed AM, Alothman AA. Characterization by Potentiometric Procedures of the Stability Constants of the Binary and Ternary Complexes of Cu(II) and Duloxetine Drug with Amino Acids. J Chem. 2019;1064942:1–13. [Crossref], [Google Scholar], [Publisher]
- Ahmed AG, Shoukry EM, Mostafa MM. Equilibrium Studies of Binary and Ternary Complexes Involving 2- Hydroxy-1- Naphthoic Acid and Amino Acids in Dioxane–Water Mixture. Egypt J Chem. 2021;64(2):623–30. [Crossref], [Google Scholar], [Publisher]
- Perrin DD, Sayce IG. complex formation by Ni and Zn with penicillamine and cysteine. Journal of the Chemical Society A: Inorganic, Physical, Theoretical. 1968;53–7. [Crossref], [Google Scholar], [Publisher]
- Maiti BK, Maia LB, Moro AJ, Lima JC, Cordas CM, Moura I, Moura JJ. Unusual reduction mechanism of copper in cysteine-rich environment. Inorganic Chemistry. 2018 Jun 29;57(14):8078-88. [Crossref], [Google Scholar], [Publisher]
- Esmaielzadeh S, Mashhadiagha G. Formation constants and thermodynamic parameters of bivalent Co, Ni, Cu and Zn complexes with Schiff base ligand: Experimental and DFT calculations. Bulletin of the Chemical Society of Ethiopia. 2017 Jul 19;31(1):159-70. [Crossref], [Google Scholar], [Publisher]
Citation: K. Asemave. Binary Stability Constants Studies of Cu and Mn-Complexes with Cysteine and Cephalexin, Adv. J. Chem. Sect. B. Nat. Prod. Med. Chem., 6(2024) 140-146